THE FUNCTION OF SQUAMATE EPIDERMATOGLYPHICS
Hobart M. Smith, David Duvall, Brent M. Graves,
Richard E. Jones and David Chiszar
Abstract: Squamate epidermatoglyphics are proposed
to function as an aid in capture, dispersal and retention of pheromones.
Snakes, lizards and amphisbaenians - hence all squamate reptiles
- are famed for the elaborate microornamentation of the epidermis, visible
under high magnification with light microscopes (Picado, 1931) but more
dramatically evident with scanning electron microscope magnifications of
up to several thousand diameters (Cole and Van Devender, 1976). The configurations
vary from mostly longitudinal ridges, as seen most abundantly in snakes
(Picado, 1931) and burrowing lizards and amphisbaenians, to spinules and
pits as in scansorial and cursorial lizards such as Sceloporus (Burstein,
Larsen and Smith, 1974). Figs. 1-4 illustrate 15 species never before depicted.
The patterns vary greatly and have been thought to be species-specific,
hence useful clues to phylogeny (Burstein et al., 1974; Price, 1982),
although Cole and Van Devender (1976) revealed evidence that intraspecific
variation is far too great to permit epidermatoglyphics to be a useful
tool taxonomically or phylogenetically. That particular problem remains
to be elucidated: the conclusions of Burstein et al. and Price cannot
be so lightly dismissed, for they took pains to eliminate factors that
might be responsible for intraspecific variation. It is likely that future
studies will restore credibility to epidermal microglyphics as clues in
systematics.
Quite aside from the possible systematic significance of epidermatoglyphics,
a question of function exists and has never been satisfactorily resolved.
Many possibilities have been suggested, conspicuous among them being a
mechanical facilitation for ecdysis (Maderson, 1970), since squamate reptiles
are unique not only in possession of epidermatoglyphics but also in being
the only vertebrates with an essentially glandless skin, the upper keratinized
layers of which are molted intact or in large pieces at frequent intervals.
The microornamentation might well facilitate the molting process in some
way, although just exactly how remains uncertain.
Creation or minimization of friction, in accordance with the habitat
and behavior of the animals, has also been proposed (Stewart and Daniel,
1973). The spinules and pits might advantageously increase friction for
crevice-dwelling reptiles, as most squamates are, at least when taking
refuge or when somnolent. The longitudinal ridges characteristic of limbless
or near-limbless squamates might facilitate movement or removal of surface
debris.
Conversion of harmful solar radiation to heat has likewise been suggested
(Porter, 1967), the fine irregularities serving as a diffraction grid.
Similarly, the structures might be useful in light refraction, producing
iridescent colors (Monroe and Monroe, 1967). There is another possibility
we here propose for the first time, and for which there is more circumstantial
evidence than exists in support of any other hypothesis: that the microglyphics
serve in the dispersal and retention of pheromones produced by scent glands
and through epidermal capillaries between scales.
Squamate reptiles have seemingly the most efficient vomeronasal olfactory
sensory system in vertebrates (Burghhardt, 1970: Duvall, King and Graves,
1982; Madison, 1977). That system is a vital means of discrimination of
food and in communication, both intra-and interspecific (Chiszar et al.,
1980; Duvall, 1979, 1982a, 1982b; Duvall et al., 1980; Kubie, Vagvolgyi,
and Halpern, 1978). Sight, hearing, tactile and taste senses are developed
in squamates to different degrees in different taxa, and in given groups
one or more may be very acute, but no sensory system is so well developed
across the board in squamates as the vomeronasal olfactory system, activated
primarily by the tongue. In no other vertebrates is the tongue used so
liberally in sampling the environment, and that attribute extends to all
members of the Squamata.
Certainly vomeronasal olfaction is equally as important as vision in
social communication, and perhaps more so, among squamates (Burghardt,
1980).
Yet squamatans (individual squamate reptiles, as opposed to collective
groups) are exceptionally impoverished in glandular sources for pheromones,
since they lack the generally distributed skin glands so characteristic
of fishes, amphibians and mammals. Other reptiles and birds are similarly
handicapped, but communication is facilitated in birds by exceptionally
acute vision, whereas reptiles other than squamates rely heavily on senses
other than olfaction for communication. They have not, incidentally, been
particularly successful groups as compared with others, with but a single
species of rhynochocephalian, some 23 of crocodilians and 250 of turtles,
as compared with some 5750 squamate species and 8750 birds.
The only well-developed scent glands remaining in squamatans are
two cloacal derivatives housed in the base of the tail in both sexes. In
only a few groups are there possible pheromonal glands about the chin (some
natricine snakes) and on the head generally (scolecophidians). For the
bulk of, if not all, squamatans, the only pheromonal source available to
them is the cloacal glands. We regard femoral and preanal pores, the escutcheon
and supraanal tubercles as dispersal, not pheromonally secretive, structures.
Yet behavioral studies show that pheromones are present on the body
surfaces generally, and that they are lost after each molt, being replaced
shortly thereafter (e.g., Kubie, Cohen, and Halpern, 1978). It seems highly
likely that the epidermatoglyphics so characteristic of squamates serve
to both disperse (by capillary action facilitated by their small size)
and retain pheromones between ecdyses. Presumably body movements aid in
spreading the pheromones produced by the anal glands over body surfaces.
The recently documented exudation of pheromones, particularly those
related to reproductive state (Duval, Guillette and Jones, 1982:212) through
the capillaries between epidermal scales (Garstka, Camazine and Crews,
1982) provides another source of olfactory material retained in place by
the epidermatoglyhphics. A possible extreme adaptation facilitating integumentary
diffusion exists in some Indian snakes noted by Malcom Smith (1943) as
having extensive bare skin overlying paired "nuchal glands" in the nape
region, where chin-rubbing commonly focuses in courting snakes. Maderson
(1965) gave an excellent account of the shedding cycle in squamates. The
lacunar layer of the epidermis separated the old epidermal generation from
the new. This layer contains many large vacuoles which
Jackson and Sharawy (1978) have shown to be filled with lipids
and incorporated into the alpha-layer as it matures. These lipids also
could be deposited on the outer layer of the new generation as proteolytic
enzymes break down the lacunar layer in preparation for ecdysis. These
may be the lipids that Warburg (1966) observed on the surface of the b
-layer (i.e. Oberhautchen?).
Maderson et al. (1978) have shown the a -layer
to be essential for restricting cutaneous water loss in reptiles, presumably,
because of lipids present in that layer, as supported by Roberts and Lillywhite's
(1980) findings that skin lipids are the determining factor in cutaneous
water loss rates, because of lipids present therein. Oldak (1976) found
that the odorous part of cloacal gland secretions are lipids which differ
between groups and are chemically stable for over two years.
All of the above provide solid evidence for presence of lipids in and
on squamate epidermis. There is also evidence for these lipids serving
pheromone function. Kubie, Choen, and Halpern (1978) have shown that estradiol
benzoate treated female garter snakes (T. radix) are more sexually attractive
after shedding and that this sexual attractiveness is transferred to penmates.
This pheromone may be lipids released from lacunar vacuoles during shedding.
However, Garstka and Crews (1981) showed that the sexual attractiveness
pheromone in garter snakes (T.sirtalis) is a lipid which moves through
the skin.
These lines of evidence appear to us to suggest very strongly that the
function of squamate epidermatoglyphics is to aid in dispersal and retention
of pheromones between molts. The acutely sensitive vomeronasal system of
these reptiles makes such a function tenable, whereas in less sensitive
vertebrates that function would be very unlikely simply because the requisite
discriminatory ability is not there. Circumstantial evidence of a functional
role in pheromone capture outweighs all evidence supporting other hypotheses.
Fig. 1
 Scanning electron microscope (SEM) photographs of selected lizard scale
surfaces. A = Cnemidophorus bacatus, x1900; specimen number not
recorded; note numerous pits. B = Sceloporus formosus, x5000; specimen
number not recorded; note profuse spicules bordering pits. C = S. scalaris,
x5000; specimen number not recorded; note short spicules on ridges bordering
pits.
Scanning electron microscope (SEM) photographs of selected lizard scale
surfaces. A = Cnemidophorus bacatus, x1900; specimen number not
recorded; note numerous pits. B = Sceloporus formosus, x5000; specimen
number not recorded; note profuse spicules bordering pits. C = S. scalaris,
x5000; specimen number not recorded; note short spicules on ridges bordering
pits.
Fig. 2
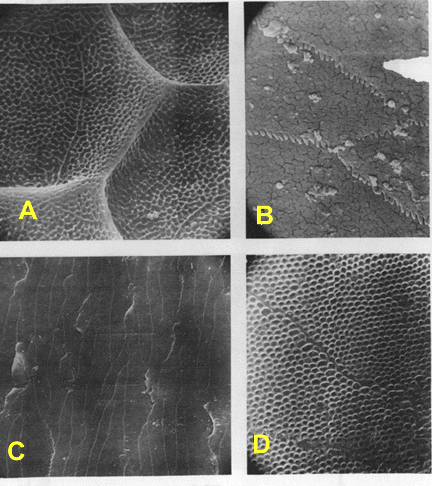
SEM photographs of selected lizard and snake scale surfaces. A = Sceloporus
grammicus microlepidotus, x5000; specimen number not recorded; note
absence of spicules bordering the pits. B = Typhlops reticulatus,
x5000; Kansas University Museum of Natural History (KU) 69820; note curious
serrations on cell margins. C = Leptotyphlops dulcis, x1000; KU
21400; cell margins are serrate. D = Corallus caninus, x5000; KU
121833; pits are unusual among snakes.
Fig. 3

SEM photographs of selected snake scale surfaces. A = Anilius scytale,
x5000; KU 121835; Price (1982) called this an echinate pattern. B =
Rhinophis drummondayi, x5000; KU 37714; another unusual pitted pattern.
C = Stenorrhina freminvillei apiata (from Yucatan), x5000; KU 70892;
another echinate pattern. D = Tretanorhinus nigroluteus, x2000;
KU 75759; a plicate pattern similar to that of Liodytes alleni (Price,
1982).
Fig. 4
 SEM photographs of selected snake scale surfaces. A = Nerodia sipedon
sipedon, x5000; KU 17757; a canaliculate pattern with crosshatching
ribs very similar to Price's (1982) illustration for Nerodia fasciata.
B = Lacicauda laticauda, x1000; KU 94559; another plicate pattern.
C = Pelamis platurus, x2000; KU 125502; plicate like the proceeding.
D = Micrurus spixi, x5000; KU 130262; echinate, much like Pseudohaje
nigra (Price, 1982).
SEM photographs of selected snake scale surfaces. A = Nerodia sipedon
sipedon, x5000; KU 17757; a canaliculate pattern with crosshatching
ribs very similar to Price's (1982) illustration for Nerodia fasciata.
B = Lacicauda laticauda, x1000; KU 94559; another plicate pattern.
C = Pelamis platurus, x2000; KU 125502; plicate like the proceeding.
D = Micrurus spixi, x5000; KU 130262; echinate, much like Pseudohaje
nigra (Price, 1982).
Literature Cited
Burghardt, G.M. 1970. Chemical perception in reptiles. pp.241-308 in:
J.W.Johnston,D.G. Moulton and A.Turk, eds., Communication by chemical signals.
New York, Appleton Century Crofts.
________1980. Behavioral and stimulus correlated in vomeronasal functioning
in reptiles: Feeding, grouping, sex and tongue use. Pp. 275-301 in:
D.Muller-Schwarze and R.M. Silverstein, eds., Chemical signals: vertebrates
and aquatic invertebrates. New York Plenum.
Burstein, N., K.R. Larson and H.M. Smith. 1974. A preliminary
survey of dematoglyphic variation in the lizard genus Sceloporus.
J. Herpet., 8(4):359-369, figs. 1-5.
Chiszar, D., S. Wellborn, M.A. Ward, K.M. Scudder and H.M. Smith. 1980.
Investigatory behavior in snakes II: cage cleaning and the induction of
defecation in snakes. Anim. Learn. Behav., 8: 505-510.
________, C.W. Radcliffe, K.M. Scudder and D. Duvall. 1982. Strike-induced
chemosensory searching by rattlesnakes: the role of envenomation-related
chemical cues in the post-strike environment. In: D.Muller-Scwarze and
R.M. Silverstein, eds., Chemical Signals III. New York, Plenum. In press.
Cole, C.J. and T.R. Van Devender. 1976. Surface structure of fossil
and recent epidermal scales from North American lizards of the genus Sceloporus
(Reptilia, Iguanidae). Bull.Am.Mus.Nat.Hist., 156(4): 451-514, figs. 1-40.
Duvall, David. 1979. Western fence lizard (Sceloporus occidentalis)
chemical signals. I. Conspecific discriminations and release of a species-typical
visual display. J.Exp. Zool., 210: 321-326.
_______. 1982a. A new question of pheromones: aspects of possible
chemical signaling and reception in the mammal-like reptiles. In: The paleobiology
of the mammal-like reptiles, by J.J. Roth, E.C. Roth, N.H. Hotton and P.D.
McLean, eds. Washington,D.C., Smithsonian Inst. In press.
_______. 1982b. Western fence lizard (Sceloporus occidentalis)
chemical signals. III. An experimental ethogram of conspecific body licking.
J.Exp. Zool., 221: 23-26.
_______, L.J. Guillette, Jr. and R.E. Jones. 1982. Environmental
control of reptilian reproductive cycles. In: Biology of the Reptilia,
Vol. 12. London, Academic Press. In press.
_______,R.L. Herskowitz and J.Trupiano-Duvall. 1980. Responses
of five-lined skinks (Eumeces fasciatus) and ground skinks (Scinella
lateralis) to conspecific and interspecific chemical cues. J.Herpet.,
14: 121-127
_______, M.B. King and B.M. Graves. 1982. Fossil and comparative
evidence for possible chemical signaling in the mammal-like reptiles. In:
Chemical Signals III, by D. Muller-Schwarze and R.M. Silverstein, eds.
New York, Plenum. In press.
Garstka, W.R., B. Camazine and D. Crews. 1982. Interactions of behavior
and physiology during the annual reproductive cycle of the redsided garter
snake (Thamnophis sirtalis parietalis). Herpetologica, 38(1):104-123,
figs. 1-9.
_______, and D.Crews. 1981. Female sex pheromone in the skin and circulation
of a garter snake. Science, 214: 681 -683.
Jackson, M.K. and M. Sharaway. 1978. Lipids and cholesterol clefts in
the lacunar cells of snake skin. Anat/ Rec., 190: 41-46.
Kubie, J.L., J. Cohen and M. Halpern.1978. Shedding enchances
the sexual attractiveness of oestradiol treated garter snakes and their
untreated penmates. Anim. Behav., 26: 562-570.
_______, A. Vagvolgyi and M.Halpern. 1978. Roles of the vomeronasal
and olfactory systems in courtship behavior of male garter snakes. J. Comp.
Physiol. Psychol., 92: 627-641.
Maderson, P.F.A. 1965. The structure and development of the squamate
epidermis. In: Biology of the skin and hair growth. A.G. Lyne and B.F.
Short, eds. Sidney, Angus and Robertson.
_______. 1970. Lizard glands and lizard hands: models for evolutionary
study. Forma et Functio, 3: 179-204.
_______, A.H. Zucker and S.I.Roth. 1978. Epidermal regeneration and
percutaneous water loss following cellophane stripping of reptile epidermis.
J. Exptl. Zoo., 204: 11-32.
Madison, D.M. 1977. Chemical communication in amphibians and reptiles.
In: Chemical signals, by D.Muller-Schwarze and M.M.Mozell, eds. New York,
Plenum.
Monroe, E.A. and S.E.Monroe. 1967. Origins of iridescent colors on the
indigo snake. Science, 159: 97-98.
Oldak, P.D. 1976. Comparison of the scent gland secretion lipids of
twenty-five snakes:implications for bio-chemical systematics. Copeia, 1976:
320-326.
Picado, T.C., 1931. Epidermal microornaments of the Crotalinae. Bull.
Anitvenin Inst. Amer., 4(4): 104-105.
Porter, W.P. 1967. Solar radiation through the living body walls of
vertebrates, with emphasis on desert reptiles. Ecol. Monogr., 37: 273-296.
Price, R.M. 1982. Dorsal snake scale microdermatoglyphics: ecological
indicator or taxonomic tool? J.Herpet., 16(3): 294-306, figs. 1-7.
Roberts, J.B. and H.B. Lillywhite. 1980. Lipid barrier to water exchange
in reptile epidermis. Science, 207:1077-1079.
Smith, M.A. 1943. The fauna of British India. Ceylon and Burma, including
the whole of the Indo-Chinese sub-region. Reptilia and Amphibia. Vol.3.
Serpentes. London, Taylor and Francis. xii, 583 pp., 166 figs.
Stewart, G.R. and R.S Daniel. 1973. Scanning electron microscopy of
scales from different body regions of three lizard species. J. Morph.,
139: 377-388.
Warburg, M.R. 1966. On the water economy of several Australian geckos,
agamids, and skinks. Copeia, 1966: 230-235.
Department of E.P.O. Biology (Hobart M. Smith and Richard E. Jones)
and Department of Psychology (David Chiszar) University of Colorado, Boulder,
Colorado 80309
and
Department of Physiology and Zoology (David Duvall and Brent M. Graves)
University of Wyoming Laramie, Wyoming 82071
Published in the Bulletin of the Philadelphia
Herpetological Society, Vol. 30 (1982) and Vol. 31 (1983-4 photos)
This site maintained by Mark
F. Miller, webmaster at herpetology.com

 Scanning electron microscope (SEM) photographs of selected lizard scale
surfaces. A = Cnemidophorus bacatus, x1900; specimen number not
recorded; note numerous pits. B = Sceloporus formosus, x5000; specimen
number not recorded; note profuse spicules bordering pits. C = S. scalaris,
x5000; specimen number not recorded; note short spicules on ridges bordering
pits.
Scanning electron microscope (SEM) photographs of selected lizard scale
surfaces. A = Cnemidophorus bacatus, x1900; specimen number not
recorded; note numerous pits. B = Sceloporus formosus, x5000; specimen
number not recorded; note profuse spicules bordering pits. C = S. scalaris,
x5000; specimen number not recorded; note short spicules on ridges bordering
pits.


 SEM photographs of selected snake scale surfaces. A = Nerodia sipedon
sipedon, x5000; KU 17757; a canaliculate pattern with crosshatching
ribs very similar to Price's (1982) illustration for Nerodia fasciata.
B = Lacicauda laticauda, x1000; KU 94559; another plicate pattern.
C = Pelamis platurus, x2000; KU 125502; plicate like the proceeding.
D = Micrurus spixi, x5000; KU 130262; echinate, much like Pseudohaje
nigra (Price, 1982).
SEM photographs of selected snake scale surfaces. A = Nerodia sipedon
sipedon, x5000; KU 17757; a canaliculate pattern with crosshatching
ribs very similar to Price's (1982) illustration for Nerodia fasciata.
B = Lacicauda laticauda, x1000; KU 94559; another plicate pattern.
C = Pelamis platurus, x2000; KU 125502; plicate like the proceeding.
D = Micrurus spixi, x5000; KU 130262; echinate, much like Pseudohaje
nigra (Price, 1982).
